Biopharmaceutical Insmed Incorporated will present data on its therapy for nontuberculous mycobacterial (NTM) lung infections, called ARIKAYCE, at the upcoming American Thoracic Society (ATS) 2015 International Conference, which will take place between May 15th and 20th in Denver. The company is presenting three abstracts on the inhalation liposomal amikacin treatment for adults with NTM associated to mycobacterium avium complex (MAC), which is currently in its late-stage of development.
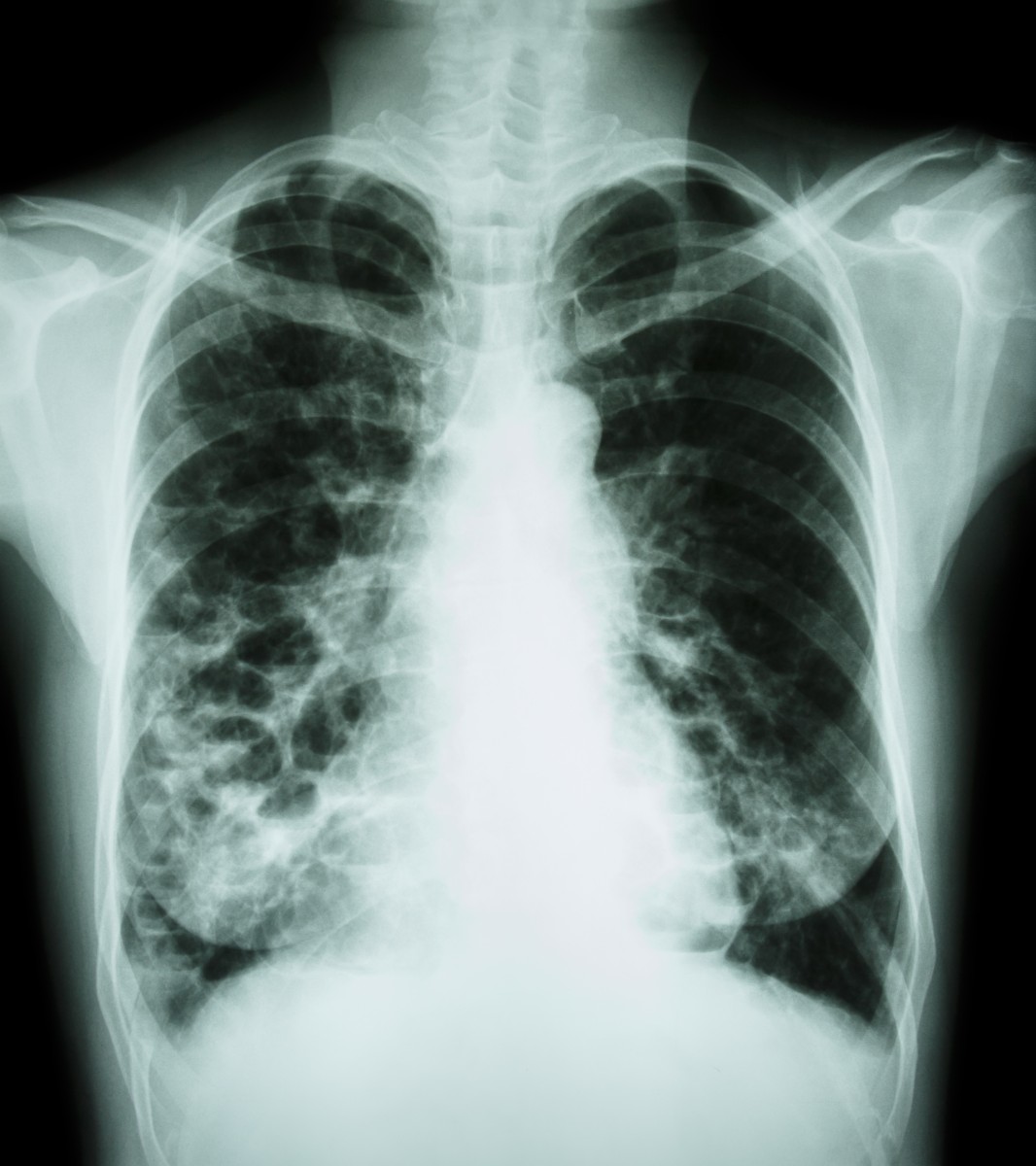
Insmed recently announced the poster session entitled “Diagnosis and Management of Nontuberculous Mycobacteria Infections,” which will be presented on Wednesday, May 20th and includes three abstracts on ARIKAYCE. The potentially chronic condition NTM lung infection is characterized by bronchiectasis and cavitary disease that can cause progressive inflammation and damage to the lungs.
Despite the fact that patients who suffer from Nontuberculous Mycobacteria Infections are frequently hospitalized, there are currently limited effective treatments for it and none is approved specifically to treat it. The treatments usually prescribed for patients who suffer from NTM lung infections are commonly multi-drug regimens, with little tolerability and particularly limited in patients with severe forms of the condition or patients who already failed to be treated by previous therapies.
ARIKAYCE, however, is expected to extent the provision of amikacin to the lungs, as well as reduce the systemic exposure. It is an antibiotic amikacin based on advanced pulmonary liposome technology, which releases biocompatible lipids endogenous to the lung that are formulated into small (0.3 micron) charge-neutral liposomes. The treatment is planned to be commercialized for once-daily administration with an optimized investigational eFlow Nebulizer System, developed by PARI Pharma.
The abstract “Subgroup Analyses of Baseline Demographics and Efficacy in Patients with Refractory Nontuberculous Mycobacteria (NTM) Lung Infection Treated with Liposomal Amikacin for Inhalation (LAI)” will be presented by its lead author, K.L. Winthrop, MD, while J.A. Biller, MD, will address the “Efficacy of Liposomal Amikacin for Inhalation (LAI) in Achieving Nontuberculous Mycobacteria (NTM) Culture Negativity in Patients Whose Lung Infection is Refractory to Guideline-Based Therapy.”
The third abstract is entitled “Analysis of Functional Exercise Capacity (Via the Six-Minute Walk Test [6MWT]) and Culture Negativity in Patients with Nontuberculous Mycobacteria (NTM) Lung Infection Refractory to Guideline-Based Therapy Treated with Liposomal Amikacin for Inhalation (LAI)” and will be presented by S.J. Ruoss, MD. All of the presentations and abstracts hosted at the ATS conference are available on the event’s website here.
Nontuberculous mycobacteria (NTM) can provoke severe lung diseases and its burden is increasing, according to the American Thoracic Society. The organisms are present in the soil and water and its prevalence is raising particularly among developing countries due to tap water and is becoming greater than tuberculosis. Most recent research estimates that there are currently about 50,000 patients who suffer from NTM lung disease.